Actuated focuses on each device’s regulatory pathway from conception through regulatory approval/clearance and into post-market surveillance. We are committed to the commercialization of our devices so that they end up in the hands of healthcare providers who can use them to improve patient outcomes.
Over the years, Actuated has built strong professional relationships with regulatory bodies in the United States (US). We interact early and often with the US Food and Drug Administration (FDA) to identify the least burdensome regulatory path and to make sure we are complying with all applicable regulations and requirements. We are registered with the FDA as a medical device.
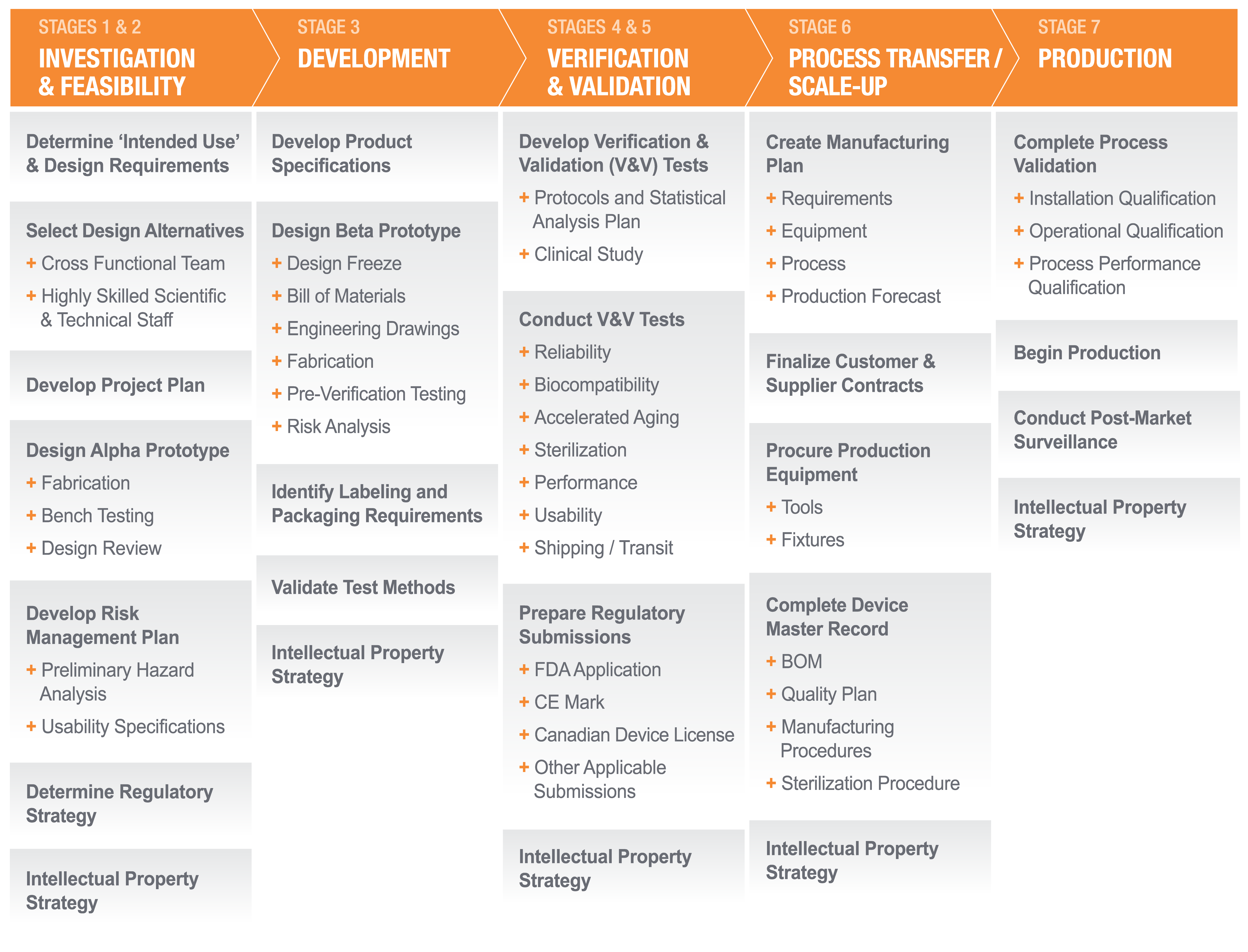
Actuated’s Seven Stage development process (see figure below) ensures that quality and safety are designed into every product. Clinician input is sought and incorporated into designs throughout this process. We have a comprehensive quality system that is compliant with 21 CFR, part 820 Quality System Regulations; Medical Devices Directive 93/42/EEC; ISO 13485:2016 – Medical Devices – Quality Management Systems – System Requirements; and ISO 14971:2007 – Application of Risk Management to Medical Devices.